Abstract
Introduction
Unrelated donor (UD)-recipient disparity for human leukocyte antigen (HLA) class I adversely affects outcome of hematopoietic cell transplantation (UD-HCT). HLA polymorphisms in the peptide antigen binding groove can affect the repertoire of presented peptides. We have recently shown that the degree of peptide divergence between mismatched HLA-DP allotypes is related to T-cell alloreactivity and clinical permissiveness after UD-HCT (Meurer et al Blood 2021). Here, we hypothesized that the clinical tolerability also of HLA class I mismatches in UD-HCT might depend on the divergence of their respective peptide repertoires.
Methods
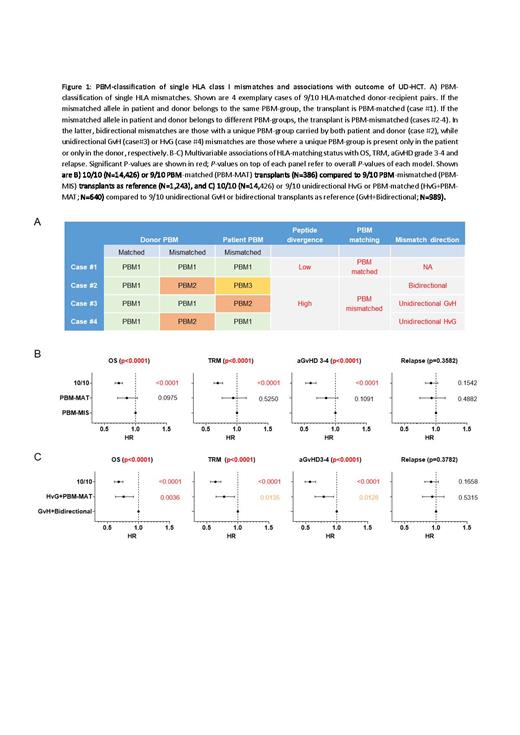
We studied 2,562 patients after 9/10 HLA-A, -B, -C, -DRB1, -DQB1-matched UD-HCT for acute myeloid or lymphocytic leukemia, or myelodysplastic syndrome, between 2008 and 2018, and 14,426 10/10 HLA-matched UD-HCT with similar characteristics. Peptide divergence of the mismatched HLA allotypes was predicted based on hierarchical clustering of experimentally determined peptide binding motifs (PBM) (Bassani-Sternberg et al Front Immunol 2018), with 21 different PBM groups identified in 122 HLA class I allotypes (44, 63 and 18 for HLA-A, -B and -C, respectively). The mismatched cohort was stratified into PBM-matches or PBM-mismatches, and within the latter into host-versus-graft (HvG), graft-versus-host (GvH) or bidirectional PBM-mismatches (Figure 1A). The primary study endpoint was overall survival (OS); secondary endpoints included treatment-related mortality (TRM), GVHD and relapse. P-value<0.01 was considered statistically significant.
Results
The available PBM data allowed us to classify 1,629/2,562 (63.6%) of our pairs. Of these, 386 (23.7%) were PBM-matched and 1,243 (76.3%) were PBM-mismatched, and in the latter, 254 (20.5%), 238 (19.1%) and 751 (60.4%) had HvG, GvH or bidirectional PBM-mismatches, respectively. Transplants were performed mainly with peripheral blood stem cells (78%), myeloablative conditioning (65%) and tacrolimus-based graft-versus-host disease (GvHD) prophylaxis (74%). About half of the 9/10 HLA-matched HCT were performed using in vivo T-cell depletion by anti-thymocyte globulin or Campath, and none used post-transplant cyclophosphamide. Multivariable analyses showed that 10/10 HLA-matched transplants had significantly higher OS, lower TRM and aGvHD 3-4 compared to 9/10 HLA-matched transplants but relapse was similar (Figure 1B,C). There were no significant differences between the PBM-matched and aggregate PBM-mismatched group (Figure 1B). In further analysis, pairs with a bidirectional or only GvH PBM-mismatch had significantly worse OS, compared to pairs in the PBM-matched group or with only a unidirectional HvG (hazards ratio [HR] 0.76, 95% confidence interval [CI] 0.63-0.92, P = 0.0036; Figure 1C). The hazards of TRM and aGvHD 3-4 were lower for the HvG or PBM-matched group compared to the reference (HR 0.78, 95% CI 0.65-0.95, P = 0.0135 and HR 0.79, 95% CI 0.65-0.95, P = 0.0126, respectively), although these were not statistically significant (Figure 1C).
Conclusion
We show that single HLA class I PBM-mismatches with high peptide divergence in the unidirectional or bidirectional GvH directions are significantly associated with worse survival after 9/10 HLA-matched UD-HCT compared to PBM-matched or unidirectional mismatching in the HvG direction. These data suggest that the mechanistic role of peptide-diversity for T-cell alloreactivity we previously observed for HLA-DPB1 disparity (Meurer et al., Blood 2021), is also a relevant to class I mismatches, providing a new rationale for selecting permissive donors in the setting of 9/10 HLA-matched UD-HCT. Avoiding class I PBM mismatches in the GvH direction is associated with better survival.
Paczesny: Medical University of South Carolina: Patents & Royalties: inventor on the ST2 bispecific antibody patent application. Lee: Amgen: Research Funding; AstraZeneca: Research Funding; Incyte: Research Funding; Janssen: Other; Kadmon: Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Syndax: Research Funding; Takeda: Research Funding.